Patexia Insight 148: ANDA Litigation Grows in the First Half of 2022
This morning we released our third annual ANDA Litigation Intelligence Report. The report covers high-level statistics related to Hatch-Waxman litigation including the analysis of the outcomes, most popular district courts, filing trends and of course, the evaluation and rankings of all attorneys, firms, judges and pharma companies based on their activity and performance.
To have the full picture of the recent activity and have a better understanding of the filing trends, we considered a filing period of five years, from July 1, 2017 through June 30, 2022. During this period, a total of 1,439 unique ANDA cases were filed.
One key observation was that the ANDA cases have been declining in recent years while it appears that this might have come to a stop as the litigation is slowly increasing based on the data for the first half of 2022. As seen in the chart below, the filing trend reached its peak in 2018 with 349 cases filed involving 488 different patents. From that year onwards, there have been several declines of 14.3%, 11.4%, and 10.9% in 2019, 2020, and 2021, respectively.
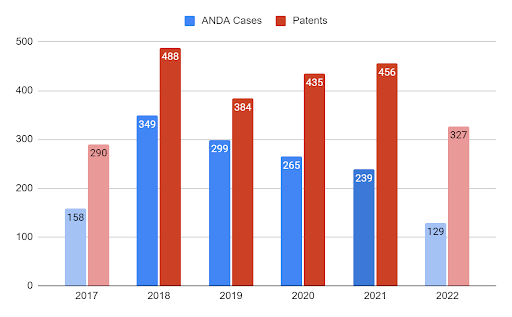
The number of patents involved follows a different pattern reaching its peak in 2018 similar to the case filings but besides the sharp decline of 21.3% in 2019, they have grown ever since. This trend continues even in 2022 as the early data shows for H1 2022. As shown in Patexia 122, the ANDA litigation trend follows quite a different pattern when compared to other areas of IP litigation such as district court patent litigation, PTAB, or ITC Section 337 investigations. While patent litigation has been increasing in recent years and its activity is influenced by factors such as market growth, innovation, competition in particular sectors, litigation funds, etc. the ANDA litigation is driven by the number of patented drugs that got the FDA approval and entered the market by brand pharmaceutical companies. Thus, in some ways, the FDA applications that are filed today may correspond to the number of Hatch-Waxman cases we will see in a couple of decades once those patents expire. If the market for the new drugs grows, they will attract competition from generic pharma companies. And that as a result, will cause Hatch-Waxman litigation down the road.
The patent litigation over the Abbreviated New Drug Applications known as ANDA litigation derives from The Drug Price Competition and Patent Restoration Act better known as Hatch Waxman Act. This act offers incentives for both brand and generic manufacturers that use ANDA cases to prove the bioequivalence of a generic drug or challenge the validity of a patent. In this list of drugs whose patents will expire in 2022, there are some entries that have generated over a billion in profit which makes them a high-value target for generic manufacturers. The first applicant to challenge a drug patent is entitled to 180 days of exclusivity by FDA against subsequent generic applicants which is a great incentive for generics. This was the case for 29 drugs up to July 2022 that got their generic approval from FDA.
To better understand the filing trends and adjust for the partial years included in the period of our study, we alter the above chart into a case/month chart as below.

The main observation is that the cases per month seem to have reached their minimum in 2021 and have had a slight increase of 1.6 cases/month for the first half of 2022. When compared with the previous year's data, there is a total of 129 cases filed during H1 2022 versus 92 cases filed during H1 2021. While this is a 40.2% growth, the entire 2022 will depend on the ANDA cases that are going to be filed in the third and fourth quarters as well. Another factor that we noticed, is the increase in the number of patents involved in ANDA.
The full 2022 ANDA Litigation Intelligence Report continues to cover other trends related to ANDA as well as the outcome analysis which is the basis for our performance rankings. A total of 415 pharma companies were involved in the 1,439 ANDA cases filed from July 1, 2017 through June 30, 2022. These were represented by 1,540 ANDA attorneys and 317 local counsel which came from 269 law firms.
Stay tuned as in the following weeks we will reveal some of the most active and best-performing pharma companies as well as several of the very best attorneys and law firms based on the data of this report.