Patexia Insight 185: From Flux to Surge: Is ANDA Litigation Rising?
We are proud to announce that tomorrow marks the release of the fourth edition of our ANDA Litigation Intelligence Report. This year's report is an in-depth compilation that includes not just data and rankings but also a thorough analysis of stakeholders involved in Hatch-Waxman activities. For the first time, we've added a dedicated chapter that highlights attorneys peer-reviewed for their performance. Additionally, we've refined our ranking methodology using machine learning to minimize external biases when calculating success scores. Our research spans a detailed five-year timeline, from July 1, 2018, to June 30, 2023, during which we analyzed 1,428 unique ANDA cases to offer a comprehensive overview of recent trends and developments.
In the realm of pharmaceuticals, an Abbreviated New Drug Application (ANDA) serves as a pivotal mechanism, facilitating the introduction of generic drug products to the market while adhering to the established patents of brand-name equivalents. These applications operate under the umbrella of the Hatch-Waxman Act, a legislative cornerstone aimed at striking a harmonious balance between the interests of drug innovation and affordability. ANDA filings, representing a strategic pathway for manufacturers, play a significant role in this landscape. These filings, while requiring rigorous compliance with regulatory standards, provide a channel for streamlined approval by leveraging the existing research data for the reference listed drug. This approach expedites the availability of generic alternatives to consumers, contributing to competitive drug pricing and broader market access.
Originating at the crossroads of pharmaceutical innovation and legal intricacies, the litigation trends within the ANDA landscape stem from the intricate interplay between generic drug manufacturers and patent holders. Unlike traditional patent litigation, where the focus often revolves around the nuances of patent validity and infringement, ANDA litigation navigates a distinct trajectory. This is evident in the filing patterns, where over the past half-decade, we've witnessed fluctuations in ANDA litigation that diverge from the trends seen in patent litigation covered by our other reports of district court patent litigation, ITC Section 337 investigations, PTAB proceedings, and even appeals at the Federal Circuit.
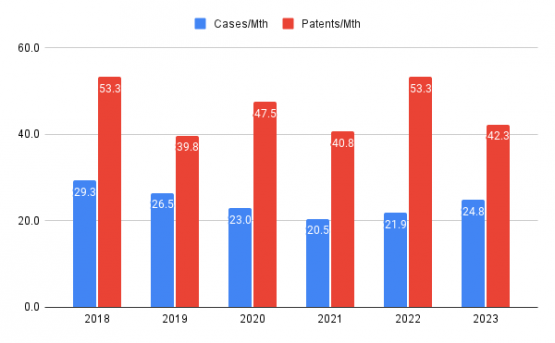
Our study focused on cases filed over a five-year period, spanning from July 1, 2018, to June 30, 2023. To account for partial years, we assessed the monthly activity and averaged the number of filed ANDA cases per month. The results were as follows: 29.3, 26.5, 23.0, 20.5, 21.9, and 24.8 for the years 2018 through 2023, respectively. These data points portray a downward trend during the initial years of the study, followed by an upward trajectory in the more recent years of 2022 and 2023.
The recent resurgence suggests a renewed interest in ANDA litigation. Several top-ranked attorneys who were interviewed anticipated a growth in the cases in the near future. Factors such as expiring patents for key pharmaceutical products, emerging markets, and past advancements in pharma research and technology might have contributed to this trend reversal. Furthermore, the evolving dynamics of pharmaceutical competition and the need for efficient strategies to navigate complex regulatory pathways may also have prompted an increased focus on ANDA litigation in recent years. As the industry continues to adapt and respond to changing demands, monitoring these trends becomes crucial for stakeholders to make informed decisions and stay ahead in this area.
The full 2023 ANDA Litigation Intelligence Report further delves into additional trends connected to ANDA cases, encompassing outcome analysis that serves as the foundation for our performance rankings, as well as insights into the most frequently encountered IPC codes, jurisdictions, and more. Throughout the period spanning from July 1, 2018, to June 30, 2023, a total of 469 pharmaceutical companies engaged in the 1,428 filed ANDA cases. These entities were represented by 1,666 ANDA attorneys and 270 local counsel, who came from 226 distinct law firms. Within our report, we have meticulously ranked these participants, considering their activity and performance in categories such as plaintiff, defendant, and overall roles.
Stay tuned for upcoming insight releases where we'll delve deeper into key insights from this report. Additionally, we're excited to unveil next week our highly anticipated PTAB Intelligence Report, which introduces a new dimension by incorporating data from PGR proceedings alongside IPRs, marking a significant milestone in this report series.
